June 5, 2025
FSMA Rule 204 Extension: What, Why, and When?
The FDA has signaled a likely extension of FSMA Rule 204 compliance to June 2028—but with no official change yet, food companies must keep preparing to avoid major traceability and regulatory risks.


What?
The U.S. Food and Drug Administration (FDA) has announced its intent to extend the compliance deadline for FSMA Rule 204 from January 2026 to June 2028. It’s important to emphasize the distinction: this is currently an intent—not an official extension. Under federal regulations, a formal change requires a structured rulemaking process. For details on the timeline, keep reading.
Why?
Why has the FDA signaled an extension? In short, the agency has acknowledged the current state of industry readiness—and it’s not ideal.
While the U.S. fresh food supply chain is efficient at moving product swiftly, it masks some significant deficiencies, particularly in traceability. FSMA Rule 204 requires the collection and sharing of Key Data Elements (KDEs) across three Critical Tracking Events (CTEs). A cornerstone KDE is the Lot or Batch Code, a unique identifier that links products to specific production runs. For example, Fresh Mozzarella produced on Monday would carry a different lot code than that produced on Tuesday.
Lot codes are crucial not only for quality assurance but also for traceability during a foodborne illness outbreak—such as listeria-contaminated spinach. Effective tracking of lot codes enables rapid identification of affected products and their origins. Unfortunately, there remains a widespread inability across the supply chain to consistently capture, store, and share lot code data.
Recognizing these gaps, the FDA made a prudent decision: to extend the timeline, allowing the industry additional time to build the systems and capabilities needed to comply fully with the Rule 204 requirements.
When?
In today's volatile market environment, much like in response to recent tariff-driven disruptions, businesses crave stability and predictability—fresh food supply chains are no exception. Companies need certainty to plan investments and operational changes. However, finalizing the extension will take time.
Here’s a high-level overview of the standard process the FDA must follow to formalize a new compliance date:
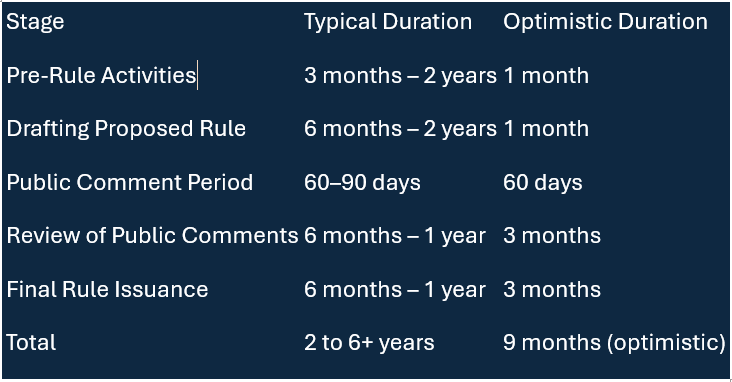
Even with an expedited process—given that the revision may involve a simple date change—it could still take several months before the new compliance date is published in the Federal Register.
Additionally, the FDA might choose to revise other parts of the rule, such as the Food Traceability List (FTL), to clarify lingering uncertainties identified by industry stakeholders. This would lengthen the process further.
Realistically, the extension may not become official until sometime in early 2026. So, can your organization afford to wait and do nothing?
Imagine if January 20, 2026, arrives without a formal extension, and shortly after, your company is involved in a food safety incident involving an FTL item. How would regulators view an organization that took no action throughout 2025 to prepare for FSMA Rule 204 compliance?
Used by the world's leading companies













































Take the First Step
Contact Tim directly to address your traceability and sustainability concerns.






